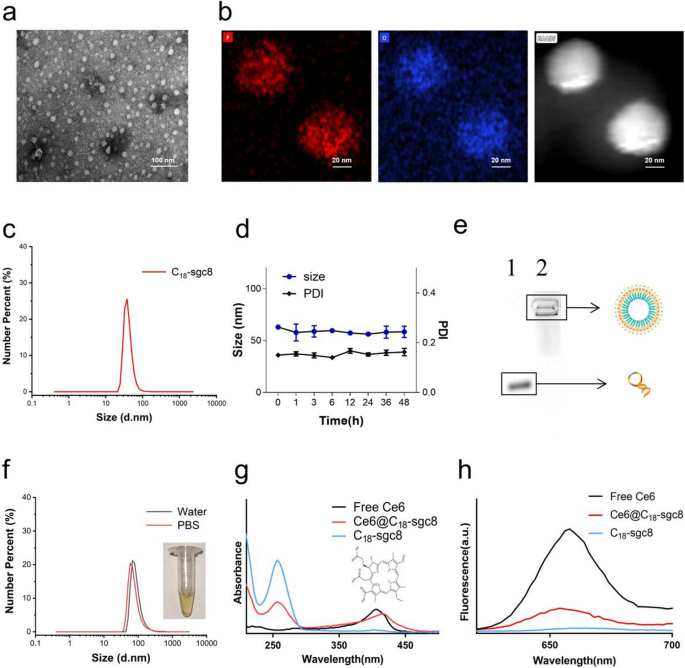
This research presents a brand new strategy to hydrophobic drug supply utilizing self-assembling nucleic acid aptamer nanomicelles. C18-sgc8 aptamer monomers have been synthesized by conjugating the maleic anhydride group within the spine of poly(maleic anhydride-alt-1-octadecene) (C18PMH) with the amino group on NH2-sgc8. The ensuing C18-sgc8 nanomicelles have been then purified by dialysis. Adverse staining transmission electron microscopy (TEM) and dynamic gentle scattering (DLS) analyses revealed that the nanomicelles have been spherical in form with a uniform measurement distribution (Fig. 1a) and a median particle measurement of 37.84 nm (Fig. 1c). As well as, TEM-energy dispersive spectroscopy (EDS) mapping revealed a excessive focus of P-element throughout the C18-sgc8 nanomicelle (Fig. 1b), confirming that C18PMH can efficiently modify quite a few DNA strands. Moreover, C18-sgc8 nanomicelles have been noticed to keep up a uniform state for as much as 48 h with a poly dispersity index (PDI) beneath 0.2 and remained secure for an extended time period (Fig. 1d and Further file 1: Fig. S1), indicating their potential as a sturdy drug supply system. C18-sgc8 is a polymer containing a lot of C18 and DNA strands linked in tandem to type secure micelle particles. As well as, as proven in Fig. 1e and 4% agarose electrophoresis confirmed the molecular weight of C18-sgc8 nanomicelles to be considerably increased than that of free sgc8. The important micelle focus (CMC) worth was decided to be roughly 0.01143 mg/mL (Further file 1: Fig. S2), demonstrating the profitable preparation of DNA-based nanomicelles. Taken collectively, these outcomes level to the promise of this nucleic acid aptamer nanomicelle supply platform for the environment friendly and efficient supply of hydrophobic medication.
Characterization of C18-sgc8 and Ce6@C18-sgc8. a TEM photographs of the self-assembly of C18-sgc8 in water to type nanomicelles. b TEM mapping photographs of C18-sgc8. c DLS measurements of C18-sgc8 displaying a median diameter of 38.8 nm. d DLS and PDI of C18-sgc8 nanometer micelles in water at 48 h. e Verification of the coupling of C18PMH and NH2-aptamers by 4% agarose gel electrophoresis. A buffer answer containing GelRed dye and NH2-sgc8 or C18-sgc8 was chosen, adopted by including to the pattern loading gap. After electrophoresis, agarose gel was used to pick out the GelRed channel within the imaging system for imaging: Lanes 1, sgc8 and a pair of, C18-sgc8. f DLS measurements of Ce6@C18-sgc8 in water (black) and PBS (crimson) micelles. g UV–vis spectra of Ce6, C18-sgc8 and Ce6@C18-sgc8. h Fluorescence spectra of Ce6, C18-sgc8 and Ce6@C18-sgc8
Subsequent, C18-sgc8 was used as a automobile to load the hydrophobic photosensitizer Ce6 by way of hydrophobic-hydrophobic interplay [28], ensuing within the formation of drug micelles, hereinafter termed as Ce6@C18-sgc8. Notably, loading with Ce6 induced a big change within the measurement distribution of the ensuing nanomicelles because the median particle measurement elevated from 37.84 to 68.06 nm in water (Fig. 1f). The UV–vis spectrum of Ce6@C18-sgc8 confirmed a attribute peak of DNA at 260 nm, together with a red-shifted peak of loaded Ce6 at 416 nm in comparison with free Ce6 (Fig. 1g). Fluorescence spectrum evaluation additional confirmed the profitable formation of Ce6@C18-sgc8 with a typical emission peak at 660 nm (Fig. 1h). Collectively, these outcomes show the profitable formation of Ce6@C18-sgc8 and spotlight its potential for drug supply.
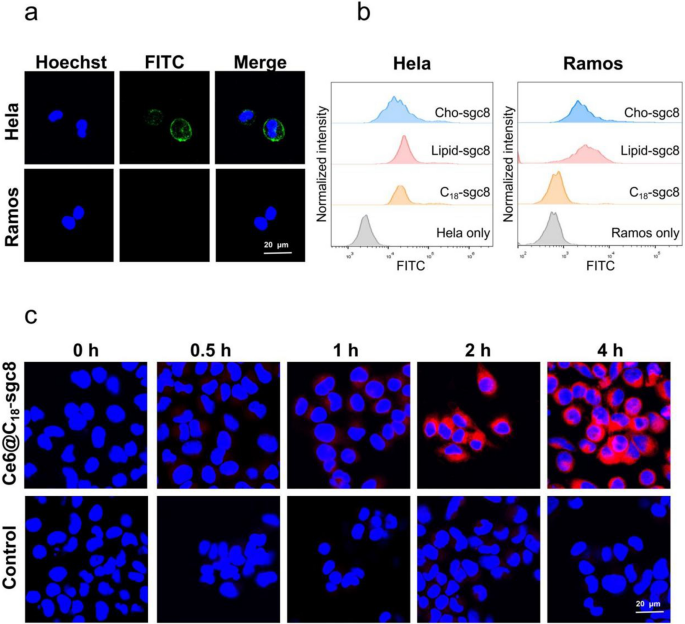
The focusing on potential of C18-sgc8 nanomicelles was additionally studied compared to different DNA nanomaterials, equivalent to lipid DNA or ldl cholesterol DNA. The latter nanomaterials have been reported to dynamically disassemble and insert into random cell membranes [29,30,31], leading to nonspecific binding to most cells. As famous above, aptamer sgc8 was chosen because the DNA fragment by its particular recognition of and binding to the extremely expressed membrane protein PTK7 on HeLa cells, however not Ramos cells [32, 33]. After incubating HeLa cells and Ramos cells with C18-sgc8 nanomicelles and imaging with the confocal microscope (Fig. 2a), it may be noticed that C18-sgc8 micelles selectively certain to HeLa cells, however not Ramos cells, beneath the identical circumstances.
a Confocal photographs of HeLa or Ramos cells incubated with C18-sgc8-FITC incubated at 4 °Cfor 1 h, adopted by observing the fluorescence of Hoechst and FITC beneath a confocal microscope. b Move cytometry histogram of HeLa or Ramos cells incubated with C18-sgc8-FITC, lipid-sgc8-FITC, or Cho-sgc8-FITC at 4 °C for 1 h. c Confocal photographs of HeLa cells incubated with Ce6@C18-sgc8 or free Ce6 at 37 °C for varied instances as indicated
To additional verify this selective binding, we incubated Lipid-sgc8, cholesterol-sgc8, and C18-sgc8 with HeLa cells or Ramos cells, respectively, for 1 h at 4 °C after which assayed their binding by circulate cytometry. Move cytometry outcomes present that C18-scg8 certain solely to HeLa cells, whereas lipid-sgc8 and cholesterol-sgc8 certain to each sorts of cells (Fig. 2b). This demonstrates that C18-sgc8 micelles have higher particular binding capability than both lipid-sgc8 or cholesterol-sgc8. This could possibly be attributed to the distinctive construction of C18-sgc8, which is a polymer with a lot of C18 and DNA strands in tandem. This construction facilitates the formation of a extra secure micelle construction that forestalls the disintegration into particular person lipid items when interacting with the cell membrane.
Subsequent, Ce6 was loaded with C18-sgc8 micelles to analyze the impact of endocytosis on the intracellular conduct of Ce6@C18-sgc8 nanomicelles. HeLa cells have been incubated with Ce6@C18-sgc8 nanomicelles and free Ce6 for various instances and imaged with confocal microscopy (Fig. 2c). Ce6@C18-sgc8 shortly endocytosed and confirmed fluorescence alerts of Ce6 within the experimental group, whereas free Ce6 had issue getting into HeLa cells. This means that C18-sgc8 micelles can successfully facilitate the entry of Ce6 into cells.
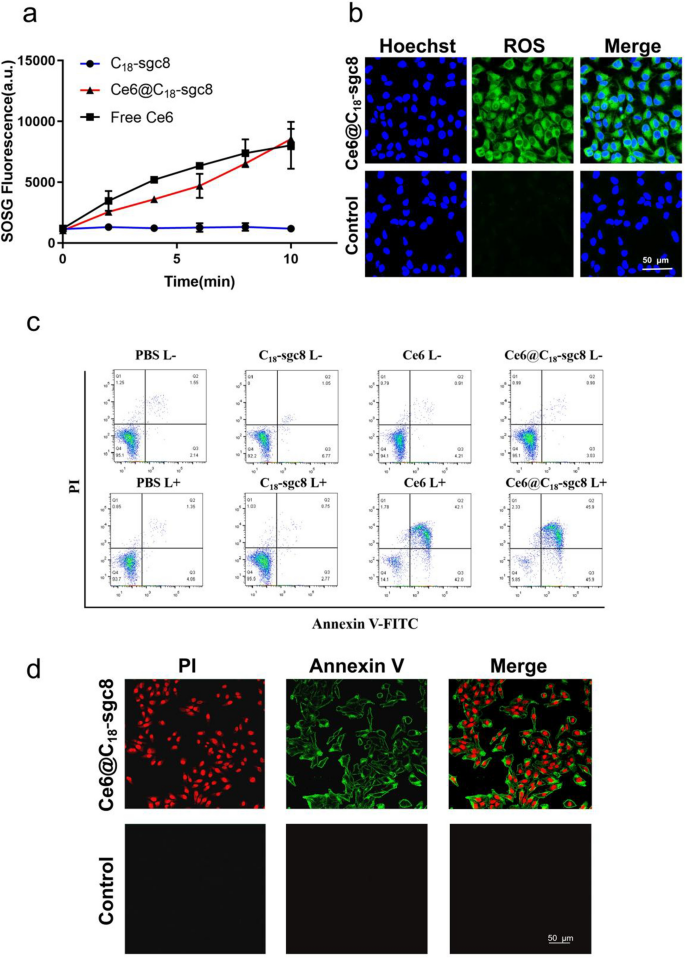
PDT combines gentle power with a drug designed to destroy cancerous and precancerous cells after gentle activation [34]. Extra particularly, throughout PDT, a photosensitizer is uncovered to particular gentle wavelengths, resulting in the conversion of oxygen in answer into extremely reactive singlet oxygen. This modification causes cytotoxicity and cell injury or apoptosis [35]. Singlet Oxygen Sensor Inexperienced (SOSG) is a single linear oxygen fluorescent probe that’s extremely selective for 1O2, and binding to 1O2 produces a fluorescence sign that may be measured by fluorescence depth [36, 37]. To analyze whether or not loading of Ce6 into C18-sgc8 impacts its potential to supply singlet oxygen, we used SOSG as a probe to detect the extent of 1O2 manufacturing. As proven in Fig. 3a, the fluorescence of SOSG doesn’t change considerably within the absence of Ce6 beneath laser radiation irradiation. Nonetheless, the fluorescence of free Ce6 and Ce6@C18-sgc8Ce6 will increase with time, indicating that Ce6 has good photodynamic properties, each earlier than and after meeting. Ce6@C18-sgc8 was additional explored for its potential to generate singlet oxygen (1O2) usually in photodynamic remedy on the mobile stage. DCFH-DA (2′,7′-dichlorofluorescein diacetate) is commonly used as a mobile 1O2 indicator to review the mobile manufacturing of 1O2 in HeLa cells in vitro [38]. As proven in Fig. 3b, after gentle radiation, vital fluorescence was produced in Ce6@C18-sgc8-treated cells as photographed by confocal microscopy, whereas management free Ce6 produced no vital fluorescence sign. This implies that C18-sgc8 may also help Ce6 to enter the cells and carry out photodynamic remedy, which could possibly be attributed to the binding of sgc8 to overexpressed PTK7, which in flip promotes drug uptake by tumor cells.
a SOSG technology of Ce6@C18-sgc8, Ce6, C18-sgc8 after 660 nm NIR laser irradiation. b Fluorescence intensities of ROS in cells after incubation of Ce6@C18-sgc8 beneath 660 nm NIR laser irradiation. c Annexin V-FITC/PI evaluation of HeLa cells after incubation with PBS, C18-sgc8, Ce6, or Ce6@C18-sgc8 beneath 660 nm NIR laser irradiation. d Confocal photographs of HeLa cells with Annexin V-FITC/PI staining after incubation of Ce6@C18-sgc8 beneath 660 nm NIR laser irradiation
PDT is a well-established method that induces apoptosis by the manufacturing of extremely reactive singlet oxygen species that may trigger cell injury and demise [39, 40]. Throughout apoptosis, phosphatidylserine is translocated from the interior to the outer facet of the cell membrane. Annexin V is a protein that particularly binds to phosphatidylserine, making it a great tool for detecting apoptosis [41, 42]. To analyze the apoptotic impact of Ce6@C18-sgc8, the Annexin V-FITC/PI equipment was used to detect apoptosis by circulate cytometric evaluation. As proven in Fig. 3c, the fluorescence sign of FITC and PI confirmed that each free Ce6 and Ce6@C18-sgc8 induced huge apoptosis (over 90%) in HeLa cells beneath gentle irradiation, suggesting that Ce6@C18-sgc8 has good antitumor potential. As well as, Annexin V-FITC/PI staining was noticed by confocal microscopy. As proven in Fig. 3d, a considerable amount of inexperienced fluorescence appeared on the membranes of most HeLa cells incubated with Ce6@C18-sgc8 after laser irradiation, which resulted from the binding of fluorescein Annexin V to phosphatidylserine on the surface of the apoptotic cell membrane. These outcomes verify that Ce6@C18-sgc8 generates singlet oxygen that linearly is dependent upon gentle irradiation in a fashion that induces apoptosis. This highlights the potential of this nucleic acid aptamer platform for photodynamic remedy.
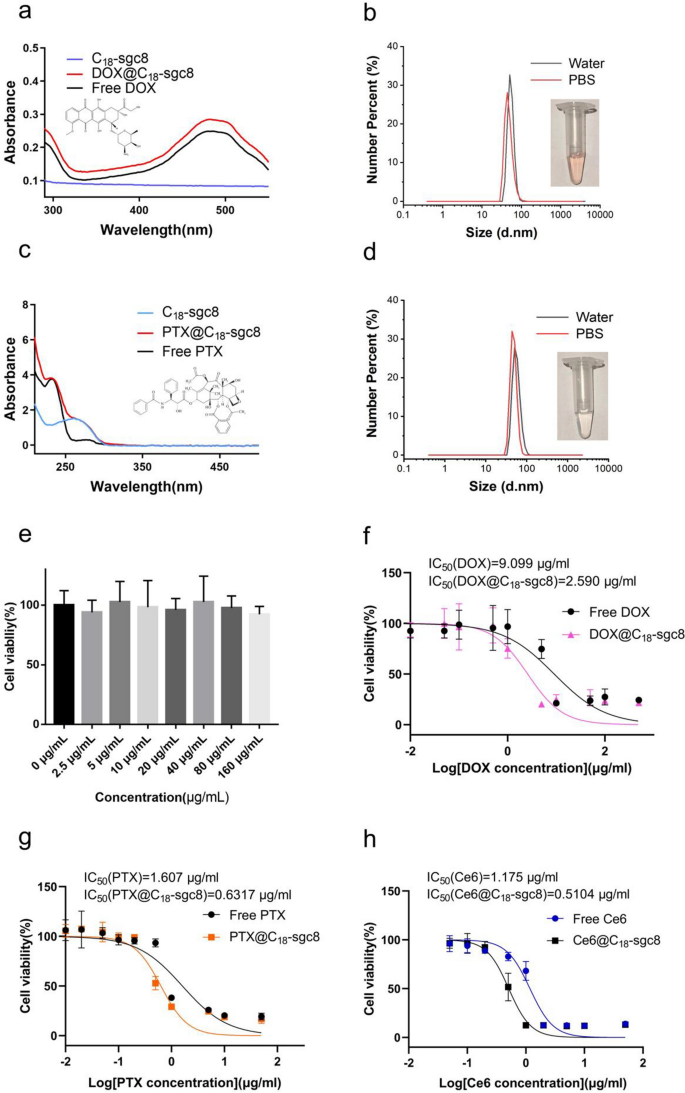
Earlier research have experimentally demonstrated that C18-sgc8 loaded with the hydrophobic photosensitizer Ce6 is efficacious in photodynamic remedy. It follows that C18-sgc8 could possibly be used as a flexible nucleic acid micelle nanoplatform for loading different medication. To discover this concept, doxorubicin (Model identify: adriamycin) and paclitaxel have been chosen to synthesize DOX@C18-sgc8 and PTX@C18-sgc8, respectively, and their particle sizes have been measured in aqueous answer or PBS. For DOX@C18-sgc8, the median particle measurement in water or PBS is about 51.75 nm and 50.75 nm, respectively (Fig. 4b), whereas the median particle measurement of PTX@C18-sgc8 in water or PBS is about 43.82 and 50.75 nm, respectively (Fig. 4d). The particle sizes of DOX@C18-sgc8 and PTX@C18-sgc8 have been each considerably bigger than the beforehand measured measurement of C18-sgc8, which may be attributed to the loading of those medication into the hydrophobic cavity contained in the micelle. As well as, fluorescence spectroscopy (Further file 1: Fig. S3) and UV-V is absorption spectroscopy have been carried out to check the absorption spectra of DOX@C18-sgc8 and PTX@C18-sgc8 with these of free DOX and PTX. Outcomes confirmed that DOX@C18-sgc8 and free DOX share an analogous attribute absorption peak at 480 nm (Fig. 4a); in the meantime, PTX@C18-sgc8 and free PTX share a attribute peak at 230.5 nm (Fig. 4c), each of which indicated that C18-sgc8 could possibly be used to load drug molecules utilizing the identical artificial technique. As proven in Further file 1: Fig. S4, the utmost drug loading effectivity of C18-sgc8 was 89.48% for Ce6 and 86.36% for PTX. These outcomes verify that C18-sgc8 is a promising platform for loading quite a lot of hydrophobic medication meant to be used in a variety of functions past PDT.
Validation of the flexibility of C18-sgc8 micelles. a UV–vis spectra of DOX, C18-sgc8 and DOX@C18-sgc8. b DLS measurements of DOX@C18-sgc8 micelles in water (black) and PBS (crimson). c UV–vis spectra of PTX, C18-sgc8 and PTX@C18-sgc8. d DLS measurements of PTX@C18-sgc8 micelles in water (black) and PBS (crimson). e Toxicity of C18-sgc8 at completely different concentrations on HeLa cells, as measured by CCK-8 assay. f Environment friendly killing of HeLa cells after therapy with completely different concentrations of DOX@C18-sgc8 or DOX. g Environment friendly killing of HeLa cells after therapy with completely different concentrations of PTX@C18-sgc8 or PTX. h Environment friendly killing of HeLa cells after therapy with completely different concentrations of Ce6@C18-sgc8 or Ce6 publicity to 660 nm gentle. Knowledge are expressed as imply ± commonplace deviation (n = 3)
To discover the potential of C18-sgc8 nanomicelles in biomedicine, experiments have been carried out to guage the impact of empty C18-sgc8 on HeLa cell viability utilizing the CCK-8 technique. First, the cell viability of empty C18-sgc8 micelles with concentrations starting from 0 to 160 µg/mL was evaluated after incubation with HeLa cells for twenty-four h. Outcomes confirmed no vital change in cell viability with rising C18-sgc8 concentrations (Fig. 4e), indicating that C18-sgc8 has good biosafety and biocompatibility. The cell-killing potential of drug-loaded micelles was additionally evaluated by figuring out IC50 values (the focus at which a substance exerts half of its maximal inhibitory impact) after incubating micelles with HeLa cells. After incubation at 4 °C for two h, the drug-loaded micelles have been washed and changed with contemporary medium for an additional 24 h incubation, adopted by performing CCK-8 assay to find out cell viability. DOX@C18-sgc8 (IC50 = 2.590 µg/mL) exhibited increased cytotoxicity in comparison with free DOX (IC50 = 9.099 µg/mL) on the similar drug focus (Fig. 4f). In the meantime, PTX@C18-sgc8 (IC50 = 0.6317 µg/mL) was extra cytotoxic than free PTX (IC50 = 1.607 µg/mL) on the similar drug focus (Fig. 4g). As well as, as proven in Fig. 4h, the cytotoxicity of Ce6@C18-sgc8 (IC50 = 0.5104 µg/mL) was discovered to be increased than that of free Ce6 (IC50 = 1.175 µg/mL) on the similar laser irradiation (1 W/cm2 for 10 min) and drug focus. This may probably be attributed to the focused binding of aptamer-modified nucleic acid micelles, which readily enter cells by way of endocytosis, facilitating drug internalization. In comparison with free medication, micelles extra effectively diffuse into cells in bigger portions inside a brief time period, leading to a stronger killing impact on tumor cells. Based mostly on the above outcomes, C18-sgc8 nanomicelles can be utilized as an all-purpose nucleic acid micelle nanoplatform for loading varied medication. Furthermore, these drug-loaded micelles exhibit enhanced cytotoxicity in comparison with free medication, making them a promising strategy for creating efficient most cancers remedy methods.
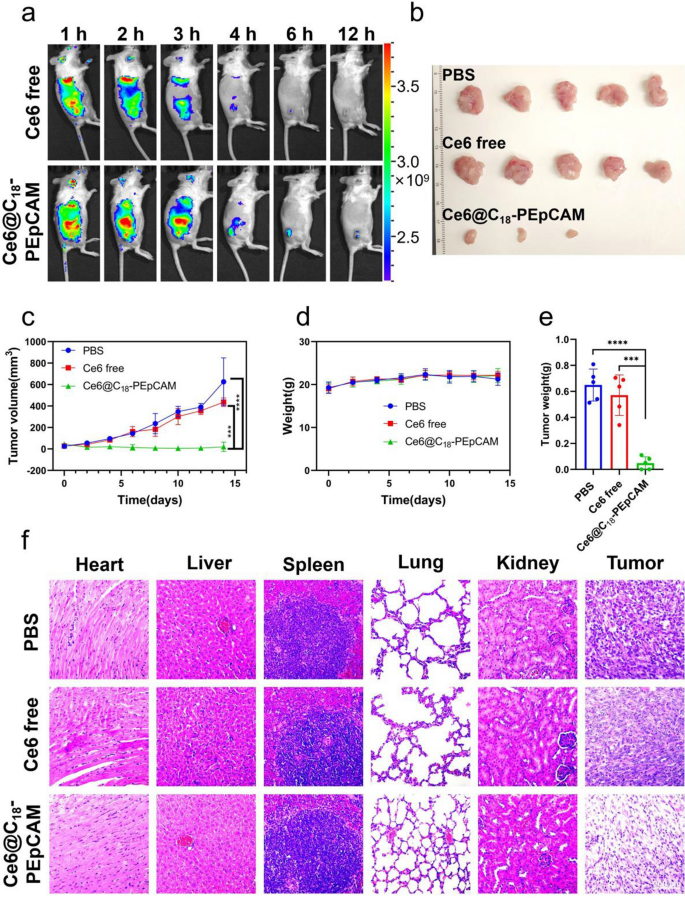
In gentle of the exceptional in vitro focused photodynamic remedy results demonstrated by aptamer-based self-assembled nanomicelles, our analysis efforts prolonged to investigating the in vivo focused photodynamic remedy results of those nanomicelles. As a proof of idea, we selected to synthesize aptamer-based self-assembled nanomicelles utilizing a nucleic acid aptamer with particular affinity for epithelial adhesion molecules identified to be extremely expressed in 4T1 cells. To optimize cost-effective, we modified a portion of polyvalent hydrophobic poly(maleic anhydride-alt-1-octadecene) by coupling it with poly(ethylene glycol) and subsequently conjugated it with the EpCAM aptamer to acquire a block copolymer named C18-PEpCAM. This block copolymer was then used within the self-assembly course of to load Ce6, ensuing within the formulation of Ce6@C18-PEpCAM. To validate the tumor-targeting efficacy of the aptamer-based micelles, Balb/c mice bearing 4T1 tumors have been administered both free Ce6 or Ce6@C18-PEpCAM, and their biodistribution was assessed utilizing in vivo fluorescence imaging. As proven in Fig. 5a, Ce6 fluorescence depth on the tumor website was considerably increased within the Ce6@C18-PEpCAM group in comparison with the free Ce6 group on the corresponding website. As well as, we harvested organs of curiosity for fluorescence imaging at 6 h after injection, as proven in Further file 1: Fig. S5. Mice injected with Ce6@C18-PEpCAM confirmed considerably elevated Ce6 accumulation throughout the tumors in comparison with mice receiving free Ce6. This indicated elevated Ce6 accumulation in tumors with Ce6@C18-PEpCAM therapy. To analyze the potential anti-tumor impact, mice have been divided into therapy teams (PBS, free Ce6, and Ce6@C18-PEpCAM) and irradiated with 100 mW/cm2 of 660 nm near-infrared gentle for 10 min after 6 h post-injection on days 0, 3, and 6, respectively. Tumor progress was monitored and after 14 days the tumors have been excised and weighed. The Ce6@C18-PEpCAM group confirmed a big delay in tumor progress in comparison with the management group (Fig. 5b, c, e and Further file 1: Fig. S6). In comparison with different teams, histological evaluation of tumor tissues confirmed in depth destruction and necrosis of tumor cells within the Ce6@C18-PEpCAM group (Fig. 5f). In the course of the 14 days, there have been no irregular modifications in physique weight or fatalities noticed in any group, indicating the biocompatibility of Ce6@C18-PEpCAM (Fig. 5d). Histological evaluation of main organs, together with coronary heart, liver, spleen, lung and kidney, additional confirmed the biocompatibility of Ce6@C18-PEpCAM (Fig. 5f).
In vivo Fluorescence imaging and PDT therapy of 4T1 tumor-bearing mice with Ce6@C18-PEpCAM. a In vivo Fluorescence imaging of mice handled with free Ce6 or Ce6@C18-PEpCAM at completely different time factors. b Images of tumors collected from mice after varied therapies on day 14. c Tumor progress curves of various therapy teams. d Physique weight of 4T1 tumor-bearing mice after varied therapies. e The tumor weight of mice after varied therapies on day 14. ***p < 0.001 and ****p < 0.0001 have been calculated by a scholar’s t-test (n = 5). f Hematoxylin and eosin (H&E) staining outcomes of main organs and tumors of mice after varied therapies